
Mechanism and Reaction of Sodium Lauryl Ether Sulfate
As a surfactant, SLES works by lowering the surface tension between ingredients like water and oil. The hydrophobic lauryl tail is attracted to dirt and oil, while the hydrophilic sulfate head is attracted to water. This allows SLES to lift away dirt and oil from surfaces and suspend it in the aqueous solution, enabling effective cleansing[1]
The hydrophobic portion of the SLES molecule consists of a 12-carbon lauryl chain derived from lauryl alcohol.[2, 3] Connected to this via an ether linkage is a series of ethylene oxide (EO) units, typically ranging from 1-4 with an average of around 2-3. The terminal end has an anionic sulfate group (-OSO3-) neutralized by a sodium cation to form the salt SLES.
In aqueous solutions, the hydrophobic tails align together while the hydrophilic heads interact with water, allowing SLES to form micelles and reduce surface tension.[4] This amphiphilic structure enables the emulsification and suspension of oils/dirt in water to be rinsed away.
Reactions
The main reactions involved in the synthesis and degradation of SLES are:
Ethoxylation: Lauryl alcohol derived from plant oils is reacted with ethylene oxide to add the desired number of EO units.[5]
Sulfonation: The ethoxylated alcohol is then sulfonated using sulfur trioxide, chlorosulfonic acid, or oleum to form a half ester of sulfuric acid.[6]
Neutralization: The acid is neutralized with sodium hydroxide to yield the final SLES product.[7]
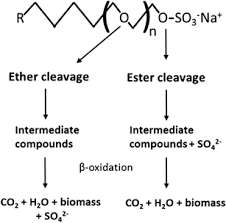
Ester cleavage: During biodegradation, SLES can undergo enzymatic ester cleavage, directly splitting off the sulfate group before the degradation of the alkyl carbon chain.[8]
Ether cleavage: The ether bonds between the EO units can also be cleaved during biodegradation, progressively shortening the ethoxylate chain.[9]
Oxidation: The alkyl and ethoxylate chains are ultimately oxidized by microorganisms, converting the carbon to CO2 and biomass. [10]
The amphiphilic structure of SLES enables its surfactant properties, while the ester, ether and carbon-carbon bonds allow it to be broken down in the environment, although the ethoxylate chain can slow degradation compared to non-ethoxylated surfactants like SDS. The sulfate head carries the anionic charge to make SLES water-soluble.
- Barra Caracciolo A, C.M., Pescatore T, Patrolecco L. Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling. Environ Pollut, 2017, 226:94-103., Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling. ., 2017.
- article, J., D. 10.1039/D3SM00276D, and R.L.H.C.A.M.R. Wilson, 2023
- Barra Caracciolo, A., et al., Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling.Environmental Pollution, 2017. 226: p. 94-103.
- Comelles, F., et al., Micellization of sodium laurylethoxysulfate (SLES) and short chain imidazolium ionic liquids in aqueous solution.Journal of Colloid and Interface Science, 2014. 425: p. 44–51.
- James Burckett St. Laurent, L.v.L., in Handbook for Cleaning/Decontamination of Surfaces, 2007, Lieva van Langenhove, in Handbook for Cleaning/Decontamination of Surfaces,.2007.
- production, C.A.-.-.-.-浙.M.s.p., Modified sulfonated product production.2001-09-13 2003-04-02.
- Almena, A., & Martín, M. (2016). Technoeconomic analysis of the production of epichlorohydrin from glycerol. Industrial & Engineering Chemistry Research, 55(12), 3226-3238., Technoeconomic analysis of the production of epichlorohydrin from glycerol.(2016).
- Negm, N.A., & Tawfik, S. M. (2013). Environmentally friendly surface active agents and their applications. Surfactants in tribology, 3, 147.,Environmentally friendly surface active agents and their applications.(2013).
- Krogh, K.A., Halling-Sørensen, B., Mogensen, B. B., & Vejrup, K. V. (2003). Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review. Chemosphere, 50(7), 871-901., Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review.(2003). .
- Jurado, E., Fernández-Serrano, M., Ríos, F., & Lechuga, M. (2013). Aerobic biodegradation of surfactants. Biodegradation-life of science, 33-61., Aerobic biodegradation of surfactants.(2013).